

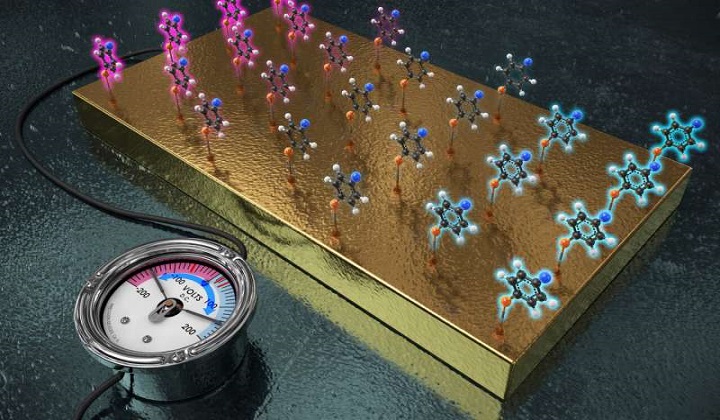
IBS and KAIST researchers employed the gold electrode and attached the target molecules onto the electrode. Just like functional groups generate diverse electronic effects, one electrode fits all reactions as the single electrode can behave like multiple functional groups just with the switch of applied voltage. The application of (+) voltage on the electrode decreased the electron density at the reaction site. Conversely, when (-) voltage was applied, the electrode acted as an electron-donating group, increasing the electron density at the reaction site. Credit: IBS
A team of researchers affiliated with several institutions in the Republic of Korea has found that it is possible to replace chemical functional groups with a gold electrode to control the reactivity of a molecule. In their paper published in the journal Science, the group describes attaching target molecules to a gold electrode to change the properties of immobilized molecules and how their technique performed when used to rate changes in the hydrolysis of certain esters.
In chemistry, functional groups are assortments of atoms that together work to attach carbon skeletons in organic molecules. All organic molecules have their own unique functional groups, which play an important role in the formation of molecules. Functional groups can also donate or take away electrons when one molecule comes into contact with another, which is how many chemical reactions occur.
Chemists have found that they can tinker with functional groups to speed up or slow down reactions to suit their needs, and because of that, functional groups play an important role in chemical synthesis. Unfortunately, developing reactions to produce desired products using functional groups has proven to be slow and difficult work. In this new effort, the researchers have found a way to replace the use of functional groups with a gold electrode to make the work easier. They simply attached molecules to a gold electrode and turned on the electricity. The technique allowed for more control over reactions by varying the amount of electricity supplied to the electrode. In such a capacity, the electrode was able to work as a "universal functional group" to inhibit or propel reactions when the researchers manipulated the amount of electricity applied to the electrode. The polarization effect of the applied potential was sufficient to exert an influence analogous to a tunable functional group.
The researchers demonstrated the utility of their technique by conducting hydrolysis and conversion experiments. They suggest the technique holds promise in other areas as well. They plan to try it with probes made of different materials to see if it might lead to better scalability.
Reference:
Heo et. al., Electro-inductive effect: Electrodes as functional groups with tunable electronic properties. Science (2020).
Source:
https://phys.org/news/2020-10-functional-groups-gold-electrode-reactivity.html

Three Universal Wiser Publisher Journals Now Indexed in Euro... Apr 23, 2024

《Sustainable and Clean Buildings》A Newly Established Journal Apr 07, 2024

Congratulations to Food Science and Engineering 1st Online E... Dec 13, 2023

Congratulations on FCE 1st Online Editorial Board Meeting, H... Dec 11, 2023

International Conference on Climate Change, Ecosystems, and ... Sep 12, 2023